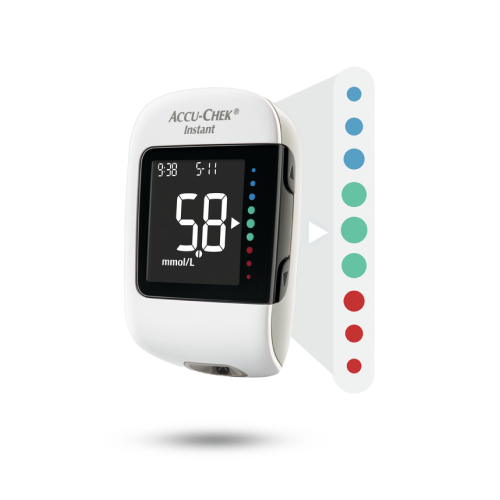
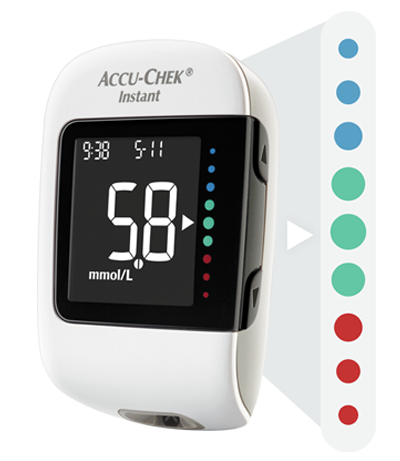
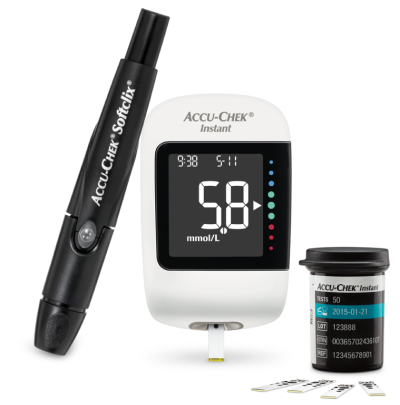
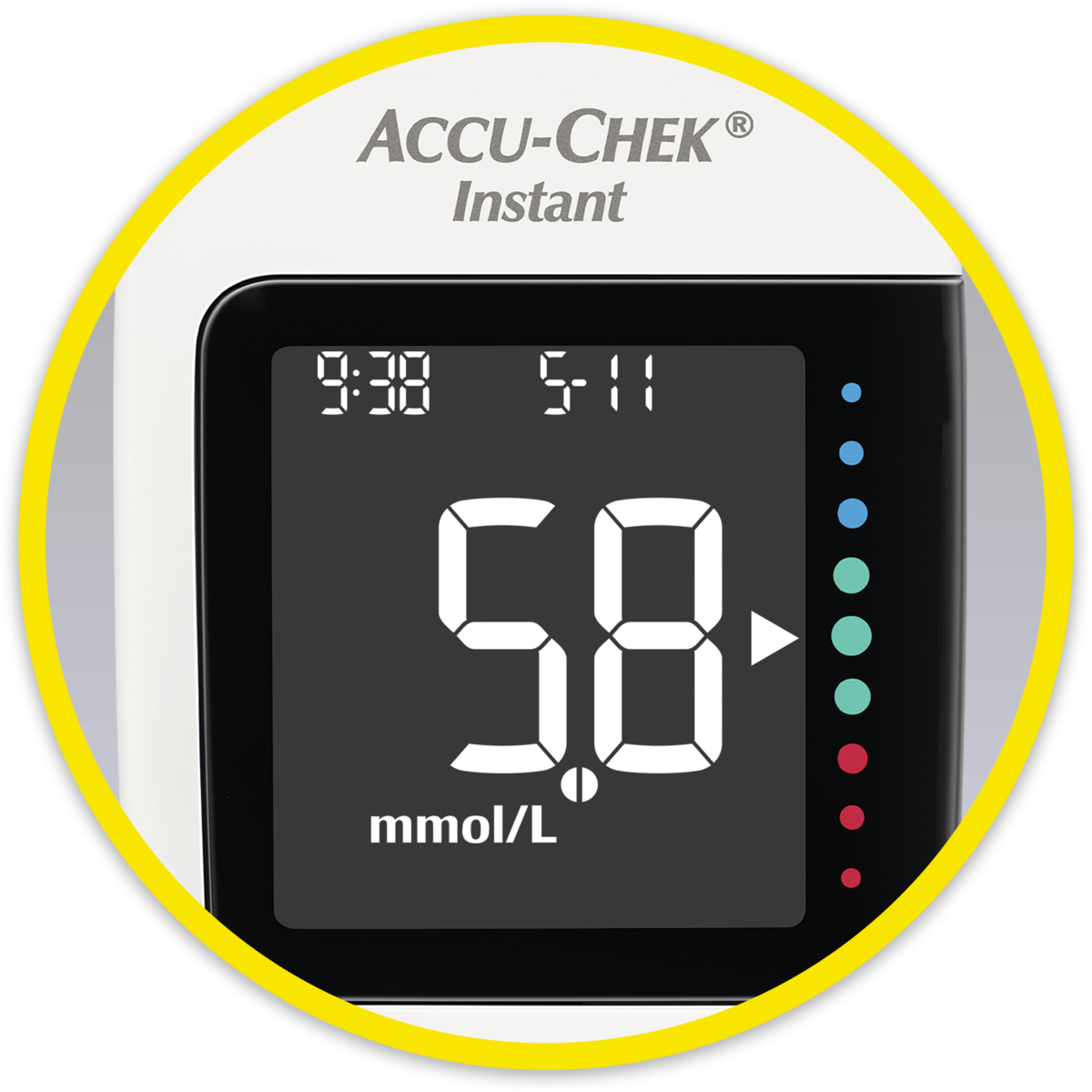
Product features of the Accu-Chek Instant system
Description
In this video you can see which product features
- MDA Reg. No: IVDC1441823-127555, GB3474823-122708
- Registered under Act 737 except for mySugr logbook which is not a medical device in Malaysia.
- MDA advertisement approval number: MDAMD 0138/2024
- Disclaimer statements e.g. This information does not promote the diagnosis, prevention or cure of diabetes. Please consult your healthcare professional for the interpretation of the result and diagnosis.