How To Use The Accu-Chek Softclix Lancing Device
Description
How To Use The Accu-Chek Softclix Device
Description
How To Use The Accu-Chek Softclix Device

The Accu-Chek Softclix lancing device can help make lancing easy. Its rotatable cap offers 11 fixed depth settings for different skin types. Clixmotion® technology minimises any painful side-to-side motion of the lancet, which is why this lancing device is virtually pain-free1 to use.
Reference:
1. Kocher, S, et al., Comparison of Lancing Devices for Self-Monitoring of Blood Glucose Regarding Lancing Pain J Diabetes Sci Technol 2009; Sep. 3(5):1136-1143.
Reference:
1. Kocher, S, et al., Comparison of Lancing Devices for Self-Monitoring of Blood Glucose Regarding Lancing Pain J Diabetes Sci Technol 2009; Sep. 3(5):1136-1143.
Control penetration with 11 depth settings
0.8 to 2.3 mm
21 g
0.4 mm (28G)
Yes (with AST cap)
Clixmotion Technology
Preparing the lancing device
| Steps | Picture Guide |
|
1. Remove cap from lancing device. |
 |
|
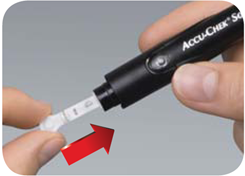
2. Insert a lancet. |
 |
| 3. Twist the sterility cap and pull gently to remove it. | 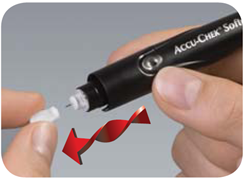 |
|
4. Replace cap. A ‘click’ sound will be heard to ensure cap is secured. |
 |
|
5. Turn dial to select depth setting (recommended setting: 1.5-3.0). The higher the number, the deeper the penetration. |
 |
Obtaining a blood sample
| Steps | Picture Guide |
| 1. Press the priming button down as shown. |  |
|
2. The transparent release button becomes yellow. |
 |
| 3. Place against the side of your fingertip. Press the yellow release button to prick. |  |
Removing a used lancet
| Steps | Picture Guide |
| 1. Aim the used lancet at a disposal bin and pull the ejector down as shown. |  |
Safety Advice:
The Accu-Chek Softclix lancing device is intended for personal use only. It must not be shared or used to collect blood in a multi-patient setting as it does not incorporate any features to guard against cross infection.
Disclaimer:
This advertisement does not promote the diagnosis, treatment, prevention or cure of diabetes. Please consult your healthcare professional for the interpretation of the result and diagnosis.
Accu-Chek Softclix and other related products are registered under Act 737.
GB63255758718